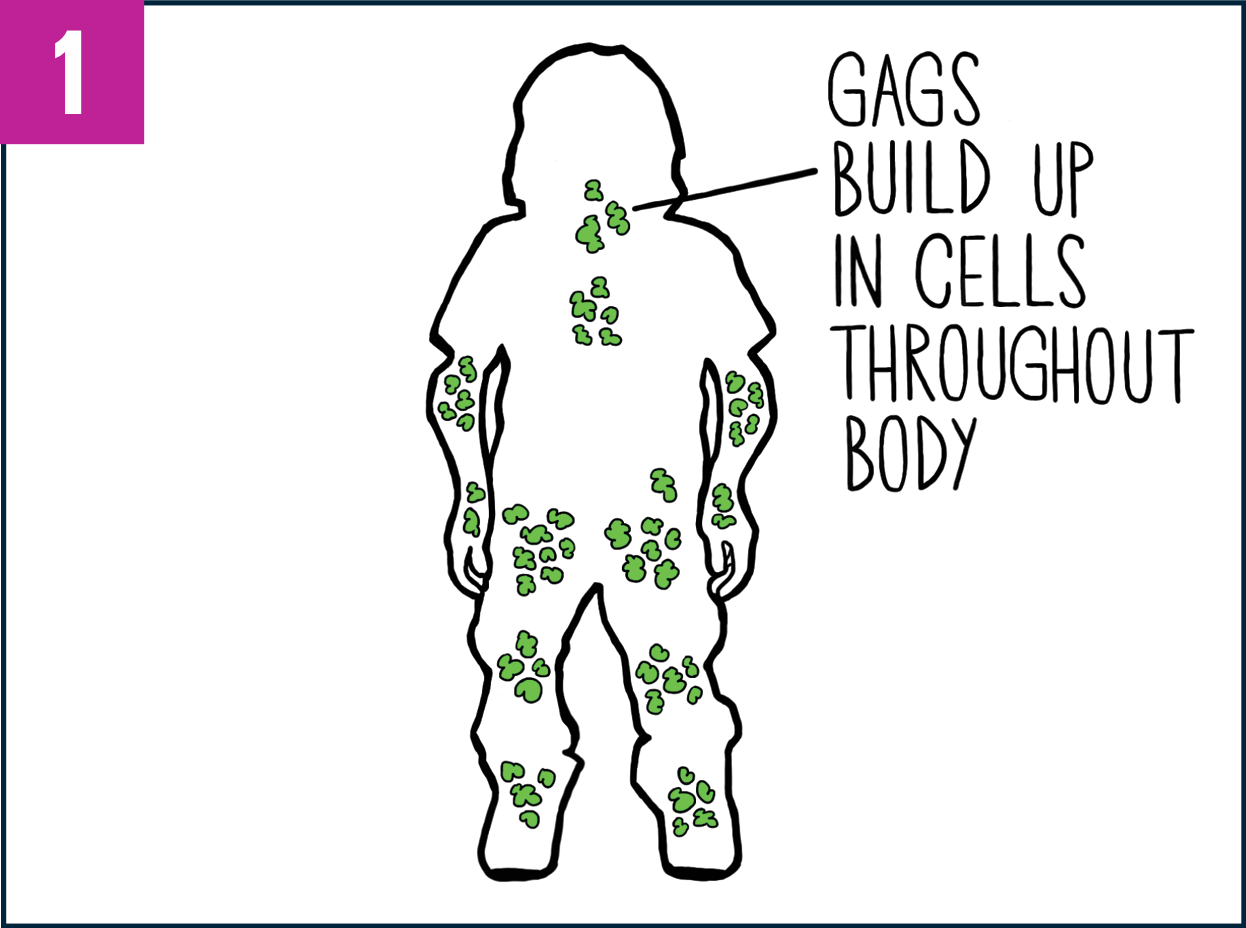
In people with Morquio A, GAGs build up in cells throughout the tissues and organs of the body, potentially causing serious problems.
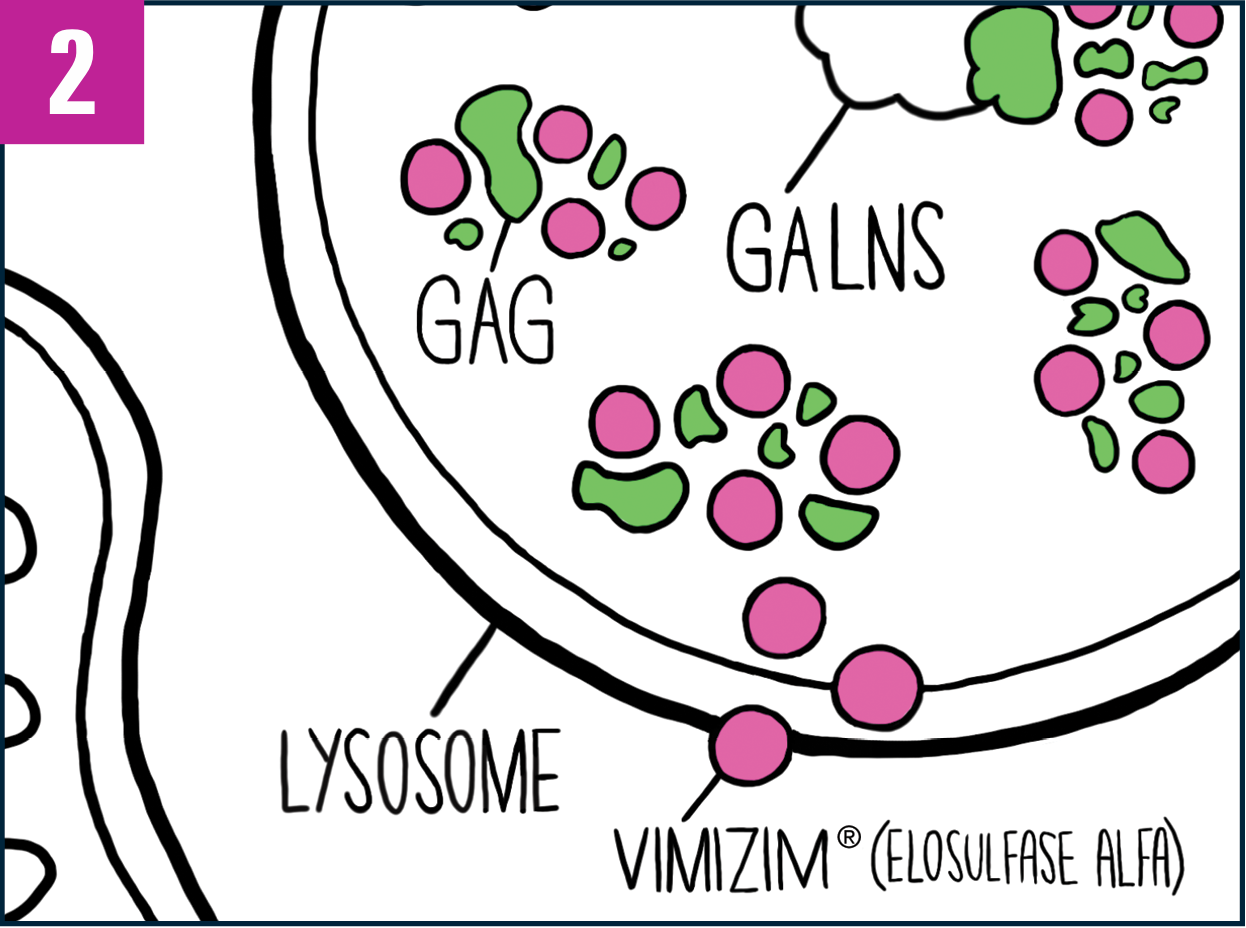
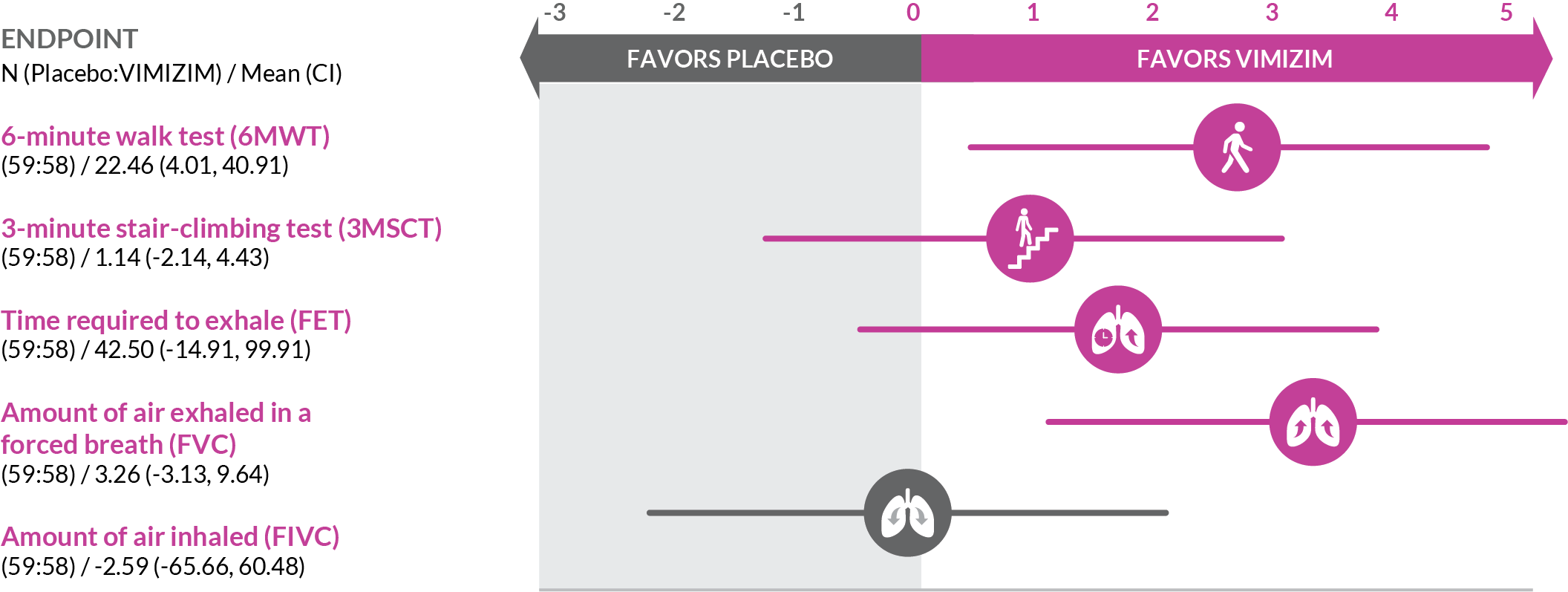
A weekly infusion of VIMIZIM helps replace the deficient GALNS enzyme your body needs to help reduce the buildup of certain GAGs.
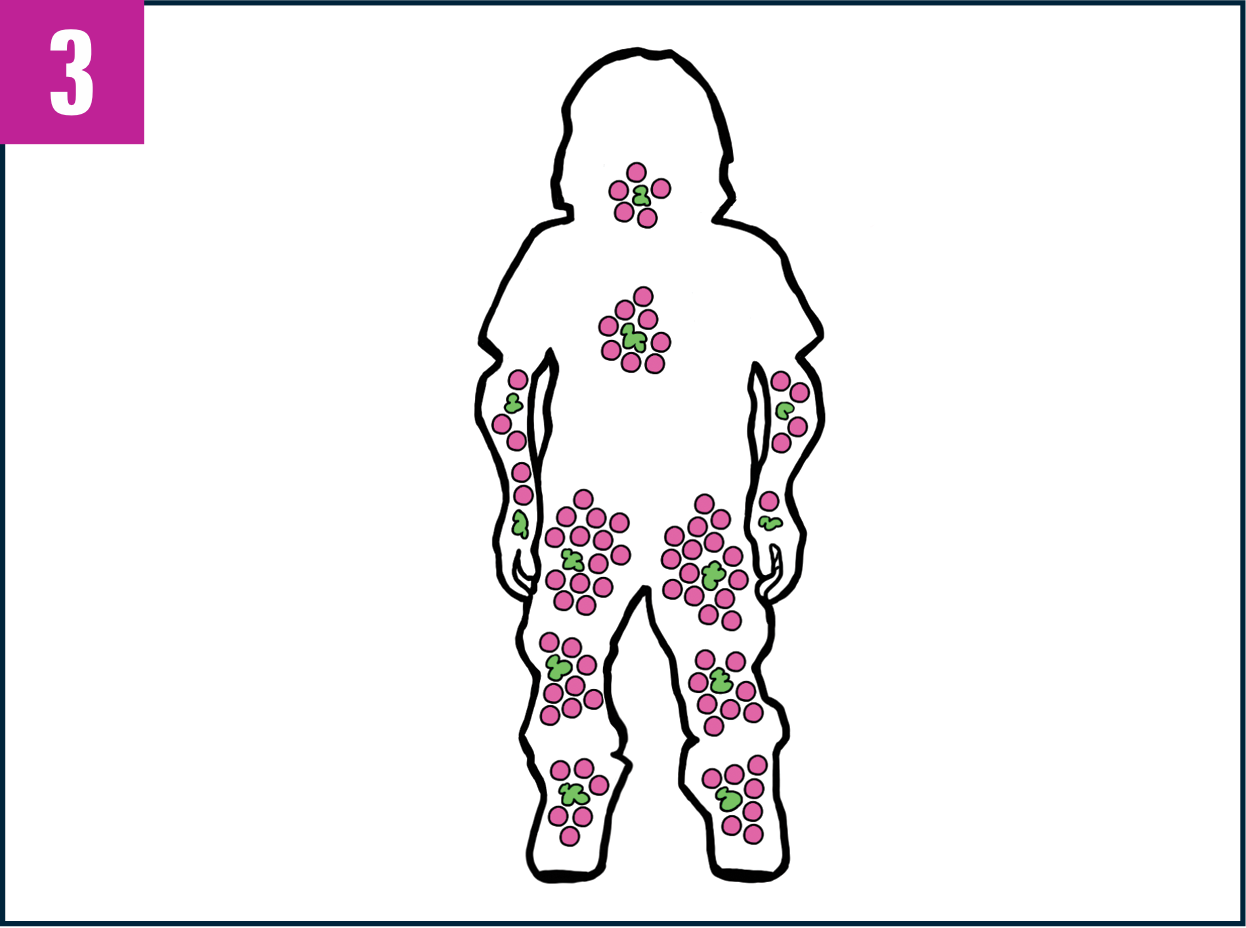
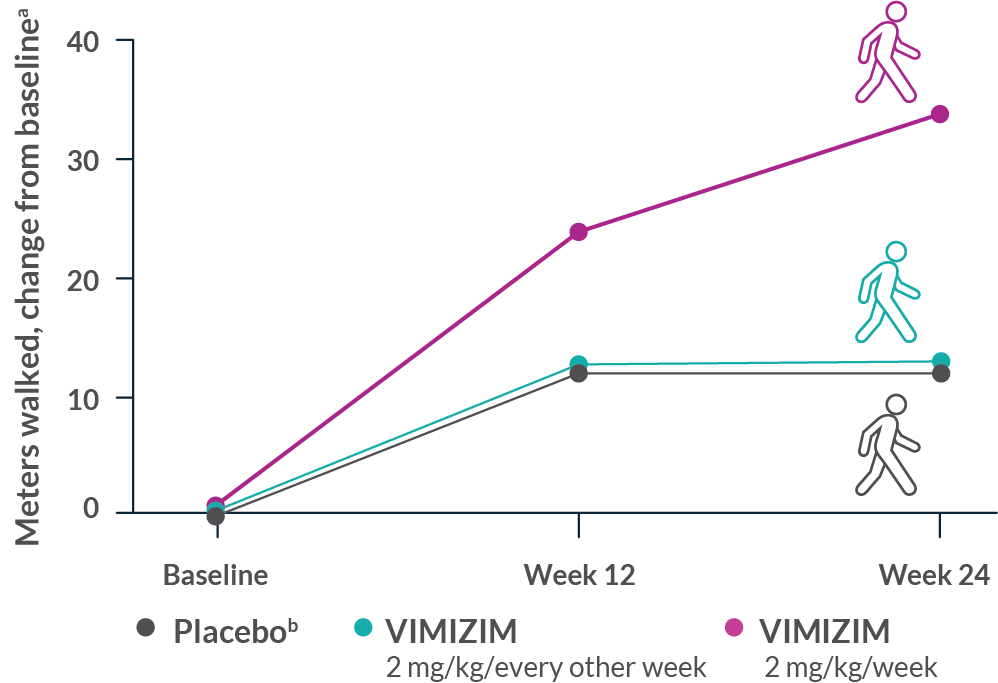
Taking VIMIZIM every week can help people with Morquio A reduce the buildup of certain GAGs.
INDICATION
VIMIZIM® (elosulfase alfa) is indicated for patients with mucopolysaccharidosis type IVA (MPS IVA; Morquio A syndrome).
IMPORTANT SAFETY INFORMATION
Life-threatening allergic reactions, known as anaphylaxis, can occur during VIMIZIM infusions. Typical signs of anaphylaxis include cough, rash, throat tightness, hives, flushing, changes in skin color, low blood pressure, shortness of breath, chest pain, and gastrointestinal symptoms such as nausea, abdominal pain, retching, and vomiting. Contact your doctor or get medical help right away if these symptoms occur during or after VIMIZIM infusions. If you have a respiratory illness, you may be at risk for a sudden worsening of your condition, and you may require additional monitoring.
VIMIZIM is a prescription medicine. Before treatment with VIMIZIM, it is important to discuss your medical history with your doctor. Tell your doctor if you are sick or taking any medication and if you are allergic to any medicines. Also tell your doctor if you are pregnant, planning to become pregnant, or are a nursing mother. Your doctor will decide if VIMIZIM is right for you. If you have questions or would like more information about VIMIZIM, contact your doctor.
Anaphylaxis can occur during any VIMIZIM infusion, as early as 30 minutes from the start and up to 3 hours after infusion, and as late into treatment as the 47th infusion. Hypersensitivity reactions have been observed as early as 30 minutes from the start of infusion but as late as 6 days after infusion.
Serious and severe reactions can happen with VIMIZIM treatment, including life-threatening allergic reactions (anaphylaxis), hives, swelling, cough, shortness of breath, and flushing. You should receive medication such as antihistamines before VIMIZIM infusions to reduce the risk of reactions. If a reaction occurs, the infusion should be slowed or stopped and you may be given additional medication. If a severe reaction occurs, the infusion should be stopped immediately and you will receive appropriate medical treatment.
If you have acute febrile or respiratory illness at the time of VIMIZIM infusion, you may be at higher risk of life-threatening complications from hypersensitivity reactions. If you use supplemental oxygen or continuous positive airway pressure (CPAP), you should have it available during your infusion in the event of a sudden reaction, or extreme drowsiness/sleep from antihistamines.
Spinal cord damage may occur due to the natural MPS IVA disease process. Signs of spinal cord injury include back pain, numbness and paralysis, and loss of bladder and bowel control. Contact your doctor immediately if you develop any of these symptoms.
The most common side effects reported during VIMIZIM infusions included fever, vomiting, headache, nausea, abdominal pain, chills, and fatigue. These are not all the possible side effects with VIMIZIM. Talk to your doctor if you have any symptoms that bother you or that do not go away.
Call your doctor for medical advice about side effects. You may report side effects to BioMarin at 1-866-906-6100 and the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088.
For more information, call BioMarin RareConnectionsTM at 1-866-906-6100.
Please see accompanying full Prescribing Information, including important warning.
US-VIM-00085 0323